As brand owners, you know how critical regulatory compliance is.
As the packaging industry continues to evolve, maintaining compliance becomes increasingly complex.
Today, we’re discussing the latest EU updates to chemical safety, specifically focusing on the EU Classification, Labelling and Packaging Regulation (CLP).
Enhanced Chemical Safety Regulations
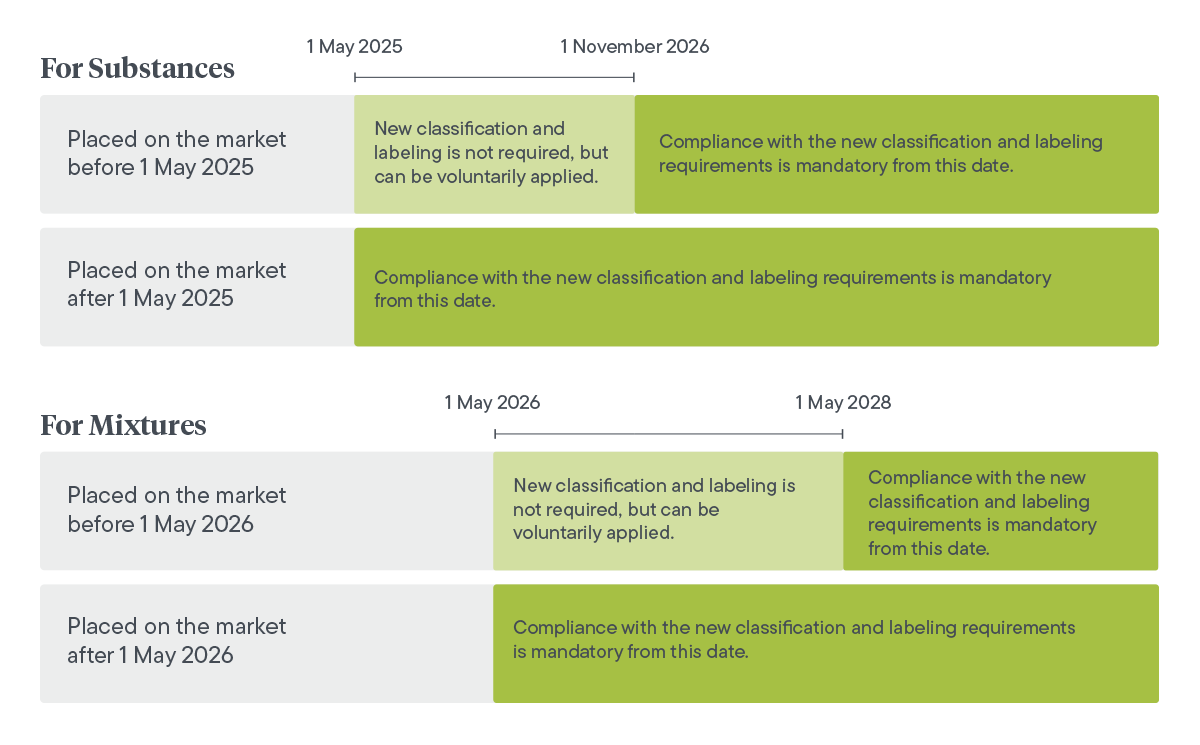
In 2023, the CLP regulation introduced new hazard classes on a voluntary basis, allowing companies to self-classify their substances and mixtures. However, starting from 1 May 2025, compliance with these classifications will become mandatory, strengthening safety measures for chemicals in the EU.
According to ECHA, the four new hazard classes addressed by the CLP update include:
- ED HH in Category 1 and Category 2 (Endocrine disruption for human health)
- ED ENV in Category 1 and Category 2 (Endocrine disruption for the environment)
- PBT (persistent, bioaccumulative, toxic), vPvB (very persistent, very bioaccumulative)
- PMT (persistent, mobile, toxic), vPvM (very persistent, very mobile)
The purpose of these regulatory updates is to ensure chemicals are appropriately labeled and classified to provide clear communication to workers and consumers throughout the European Union.
Based on the United Nations’ Globally Harmonized System (GHS), these updates provide high levels of environmental health protection to the public.
Overall, they improve safety, transparency, and consumer awareness.
To be compliant, brands must now classify hazardous chemicals on their labeling and packaging before releasing their products to market.
Keep reading to understand how CLP impacts brands and how to prepare labeling and packaging for compliance.
Major Revisions to the EU CLP Regulation
Effective December 10, 2024, changes to the CLP regulation require specific updates to labeling and packaging.
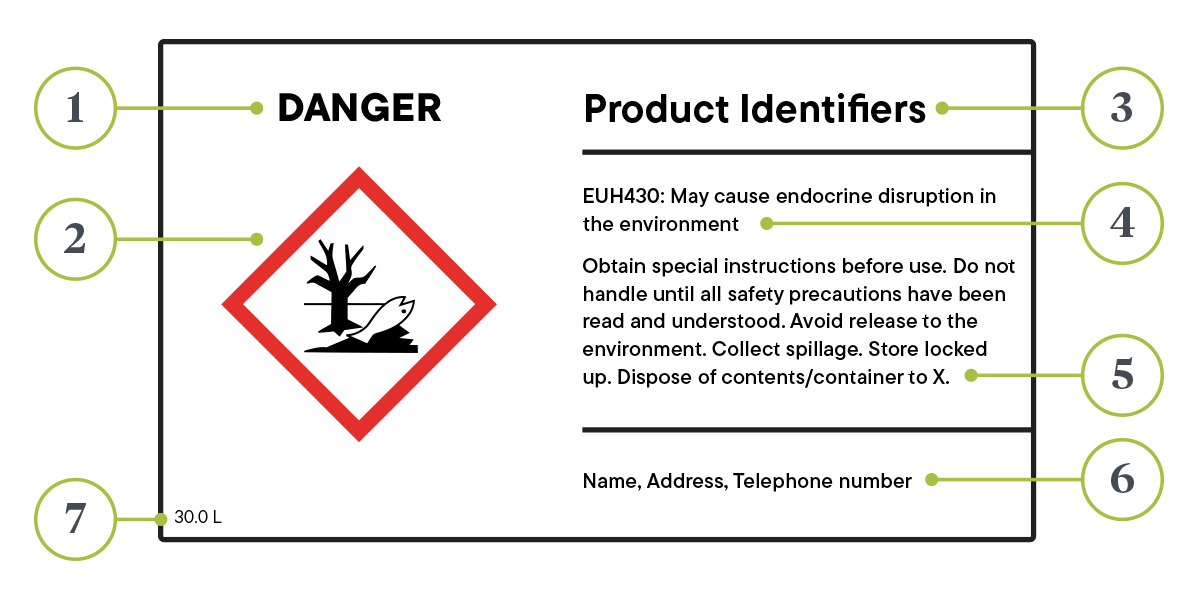
As stated in the ECHA, the label must be attached to at least one of the packaging’s surfaces. It must also include:
- The name, address and telephone number of the supplier
- The nominal quantity of a substance or mixture in packages made available to the public (unless this quantity is specified elsewhere on the package)
- Product identifiers
- Where applicable, hazard pictograms, signal words, hazard statements, precautionary statements and supplemental information required by other legislation